Our Focus
Serac Healthcare is focused on bringing effective imaging tools to the market to accelerate diagnosis, improve monitoring, and deliver earlier and more effective treatment decisions for patients. Our primary focus is on conditions which are characterised by abnormal angiogenesis (new blood vessel formation) with major unmet clinical needs: endometriosis and inflammatory arthritis.
Problem
Endometriosis: the invisible pain
Endometriosis is estimated to affect about 10% of women of childbearing age. In this condition, cells similar to the ones lining the uterus (the endometrium) grow elsewhere in the abdomen and sometimes even farther afield. These cells react to the menstrual cycle each month and also bleed.
However, there is no way for this blood to leave the body. This can cause inflammation, pain, and the formation of scar tissue. Endometriosis can have a significant impact on a person’s life in a number of ways, including:
- Chronic pain
- Fatigue
- Depression
- An inability to conceive
- Poor attendance at school/ higher education
- Problems with a couple’s sex life/relationships
- Difficulty in fulfilling work and social commitments
- 50% of women with fertility issues also have endometriosis


Solution
New Diagnostic Potential for Endometriosis
There is currently no cure for endometriosis but treatments can reduce the severity of symptoms and improve the quality of life. A definitive diagnosis requires laparoscopy – an operation in which a camera (a laparoscope) is inserted into the pelvis via small cuts in the abdomen.
However, appropriate treatment can only be delivered following an accurate diagnosis and there is currently an average of nine years from onset of symptoms to diagnosis.
There is, therefore, a clear need for a simple, rapid, robust, reliable, readily accessible, and cost-effective technique to diagnose endometriosis and guide treatment decisions. Such a test could also play an important role in assisting the development of future therapies.
The growth of endometrial tissue is dependent upon new blood vessel formation (angiogenesis) which studies have shown can be imaged with 99mTc-maraciclatide. This raises the possibility that 99mTc-maraciclatide has the potential to be a non-invasive diagnostic for endometriosis which could benefit millions of women worldwide. The Phase II clinical study to evaluate the utility of 99mTc-maraciclatide is underway.
Problem
Identifying and Monitoring Inflammatory Arthritis is Challenging
Rheumatoid arthritis (RA), psoriatic arthritis (PsA), and axial spondyloarthritis (AxSpA) are chronic, progressive, painful, incurable conditions in which the body’s own immune system attacks the joints. If untreated they can result in irreversible joint damage and permanent disability.
Multiple therapies are available that can slow or even halt disease progression but it remains a challenge to identify which treatment is best for each individual patient:
- There is no definitive blood marker, such as blood glucose levels in diabetes
- X-rays show the damage that has already been done to the joints
- Full clinical assessments require significant hands-on time with a rheumatologist and symptoms do not always correlate with the underlying disease process
- MRI is expensive and not readily accessible
- Ultrasound is highly dependent upon the skill of the operator and is time-consuming
Rheumatologists currently lack a fast, easy, readily available, accurate, and cost-effective tool to assess inflammation in the joints of patients and inform treatment decisions.
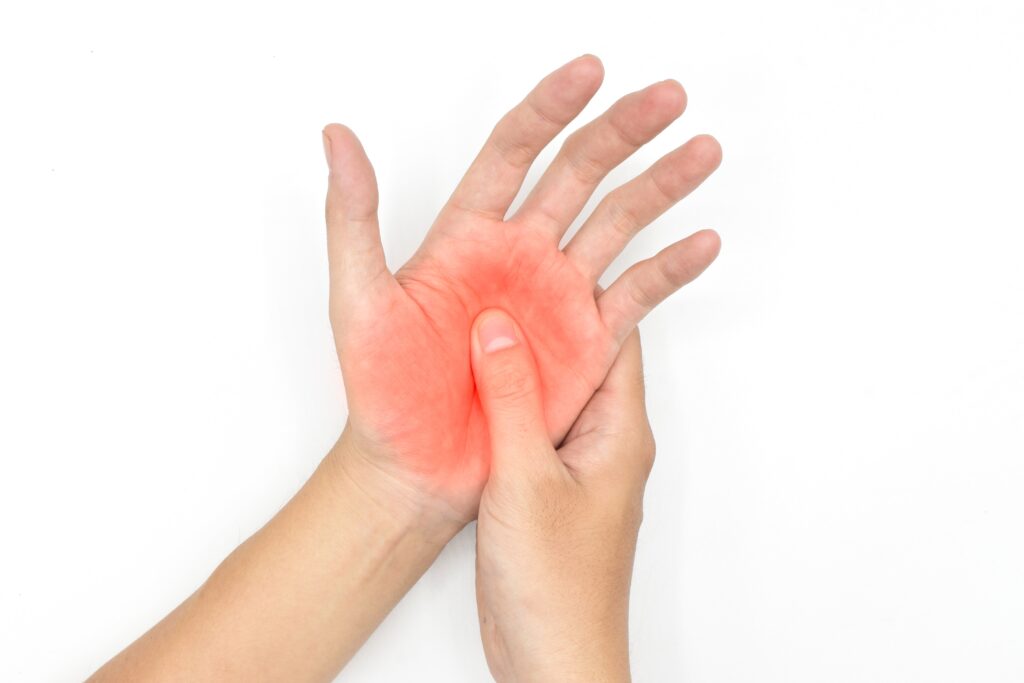

Solution
Whole Body Imaging in a 20 Minute Scan
Clinical studies are exploring whether 99mTc-maraciclatide could allow rheumatologists to see inflammation in the joints which could help them deliver the right treatment to the right patient at the right time (personalised medicine) potentially leading to improved outcomes and better quality of life.
99mTc-maraciclatide planar imaging has the potential to image the whole body, highlighting total inflammatory load in the joints in a 20-minute scan, aiming to produce images that are easy to interpret even to the untrained observer.
A Phase III study in this indication will commence in 2024.
R&D Pipeline
99mTc-maraciclatide is in development for the following indications:
Indication | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
Endometriosis | Completed | Ongoing – Awarded Fast Track Designation for SPE | |
Inflammatory Arthritis | Completed | Completed | Recruiting in RA and PsA |
Interstitial Lung Disease | Completed | Ongoing | |
Other indications | Completed | Assessing |

